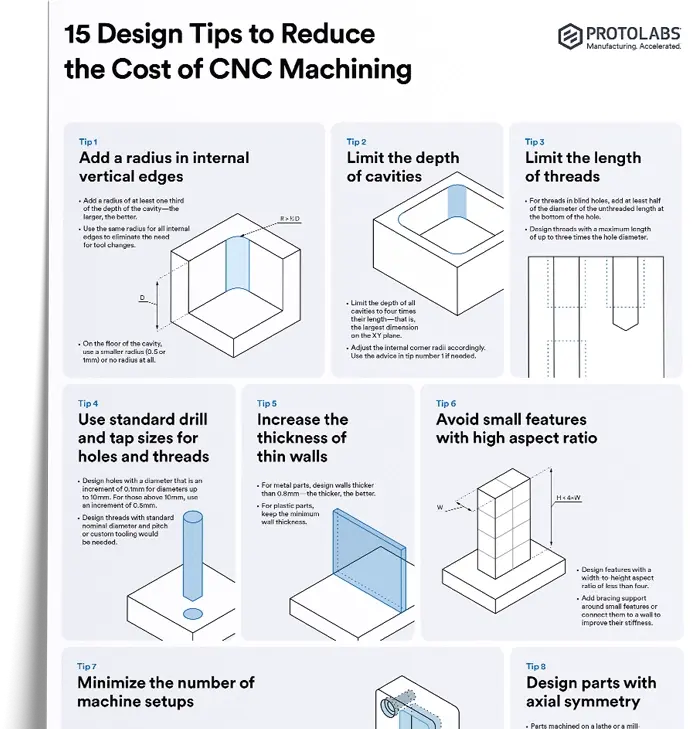
What are metalloids?
Metalloids are a group of elements that have properties of both metals and nonmetals. On the periodic table, they sit along the "stair-step" line separating the two categories. Metalloids straddle the versatile middle ground between metals, which are malleable and highly conductive, and nonmetals, which are brittle and insulating. They include elements like silicon, boron, arsenic, and antimony.
Metalloids aren’t plastics or polymers—they’re pure elements that manufacturers rely on for their distinct ability to adapt. For example, silicon’s semiconductor properties make it the backbone of the tech industry, while boron enhances the strength of lightweight composites used in aerospace. These elements are like the Swiss Army knives of materials—flexible, reliable, and useful in surprising ways.
Physical properties of metalloids
Metalloids share some properties with metals, but each comes with its own quirks:
-
Shiny yet brittle: Metalloids often look metallic, but they’re more likely to shatter under pressure than bend like traditional metals.
-
Lighter than metals: Their lower density makes them a top choice for applications where weight matters, like portable electronics.
-
Versatile forms: Some, like boron, appear as powders, while others, like silicon, are crystalline solids.
Picture the lightweight but durable materials in your favorite gaming console or the reflective, brittle wafers used in solar panels. Those are metalloids in action.
Chemical properties of metalloids
Metalloids really can’t pick a side. They have a flexible chemical nature, allowing them to exhibit characteristics of both metals and nonmetals depending on the chemical reaction they’re involved in. For example, metalloids can lose electrons in chemical reactions, similar to metals, forming positive ions (cations). But they can also gain electrons, like nonmetals, forming negative ions (anions) or covalent bonds. This adaptability is why they’re used in so many industries.
-
Amphoteric behavior: Metalloids can react with both acids and bases, giving them wide chemical versatility. For example, boron reacts with fluorine (a nonmetal) to form boron trifluoride, a compound critical in chemical synthesis.
-
Alloy-friendly: Metalloids often enhance the properties of alloys, making them harder, stronger, or more heat-resistant. For example, antimony is added to lead in car batteries to improve durability, and silicon can strengthen aluminum alloys for aerospace applications.
Electrical and thermal conductivity
Metalloids are top picks for environments where conductivity needs to be controlled. They don’t conduct electricity as freely as metals, but they outperform nonmetals.
-
Electrical conductivity: Silicon’s semiconductor properties are great for computer chips, smartphones and solar panels. Pure silicon’s conductivity is quite low , but it gets a big boost when doped* with phosphorus or boron.
-
Thermal conductivity: Metalloids like boron and silicon can withstand and transfer heat efficiently, perfect for high-performance electronics.
To put this into practice, in laptops, silicon is used in processors to manage electrical currents, while boron is used in components like heat-resistant glass covers for high-temperature stability. So the next time your laptop doesn’t overheat during a marathon work session, you can thank the metalloid-enhanced components inside.
*Doping involves intentionally introducing small quantities of impurities into a material to change its electrical properties.
Comparing metalloids with metals and nonmetals
If you’re wondering how metalloids stack up against metals and nonmetals, here’s the breakdown:
-
Metals: Highly conductive, ductile, and dense. Think of the steel in skyscrapers.
-
Nonmetals: Poor conductors, brittle, and lightweight. Like the plastic housing on your earbuds.
-
Metalloids: Balanced properties—semiconducting, moderately strong, and adaptable. Perfect for the tech inside those earbuds.
Metalloids aren’t as strong as metals or as flexible as polymers, but their versatility makes them ideal for specific tasks.
Metalloids commonly used in manufacturing
The complete list of metalloids is hard to pin down because there isn’t one universally recognized definition for metalloids or ‘semi-metals’. The number of elements that qualify ranges from six to 11, depending on who you ask. Generally, these are the six most widely agreed upon elements regarded as metalloids:
| Metalloid | Tensile Strength (MPa) | Thermal Conductivity (W/m·K) | Electrical Conductivity (S/m) | Key Applications |
|---|---|---|---|---|
| Boron (B) | 3000 | 27 | 1.00E-06 | Aerospace composites, fiberglass, armor |
| Silicon (Si) | 7000 | 149 | 1e-3 (undoped) | Semiconductors, solar panels, microchips |
| Germanium (Ge) | 110 | 60 | 2.2e-3 (undoped) | Fiber optics, infrared optics, solar panels |
| Antimony (Sb) | 55 | 24 | 1.67E+06 | Flame retardants, lead-acid batteries, alloys |
| Tellurium (Te) | 70 | 2.35 | 2.38E+06 | Thermoelectric devices, solar panels, steel alloys |
| Arsenic (As) | 20 | 50 | 3.30E+04 | Toxic and rarely used. Occasionally applied in gallium arsenide semiconductors & specialized alloys |
Applications of metalloids in manufacturing
Metalloids are most commonly used in CNC machining, where materials like silicon and boron are directly machined for precision components in electronics, aerospace, and energy industries. In injection molding, metalloids like antimony appear as additives (e.g., flame retardants) in molded plastics for construction and consumer goods. The use of metalloids in 3D printing and sheet metal fabrication is more niche, often involving experimental applications (e.g., silicon-based resins) or as part of alloys rather than standalone materials.
While there are six metalloids used in manufacturing, three stand out as the most valuable players: silicon, boron and antimony. These are the unsung heroes of modern manufacturing, quietly making your favorite gadgets and tools work.
-
Silicon: Found often in consumer electronics, silicon is used in smartphones, computers, and renewable energy systems like solar panels. It can switch between insulating and conducting states when doped, which is why it’s ideal for microchips and solar cells. Because of its semiconducting properties, it can also be used for controlling electrical currents, which you need for the operation of electronic devices and energy systems.
-
Boron: Boron strengthens aerospace composites and can improve the toughness of glass cookware. Its lightweight yet rigid structure makes it perfect for reducing weight in aircraft while maintaining strength, and its high heat resistance ensures durability in glass used for cooking or industrial applications.
-
Antimony: Antimony provides flame retardancy in industrial machinery, construction and textiles.
These materials aren’t just functional—they’re essential for creating innovative, durable products.
Metalloids in modern technology
Every high-tech gadget you use likely has metalloids working behind the scenes. Silicon is the superstar of the semiconductor world, powering everything from gaming accessories, like Formify's custom mousepad for pro gamers, to efficient electric vehicle chargers, such as those developed by LEKTRI.CO. Boron’s heat resistance makes it critical in borosilicate glass used for aerospace windshields and re-entry shields on spacecraft. Antimony, widely used in flame-retardant plastics and coatings for electrical wiring, helps promote safety in construction and industrial machinery.
The potential of metalloids in manufacturing
Metalloids are just getting warmed up. Their unique properties position them as key players in future innovations:
-
Smart materials: With the boom of specialized 3D printing materials and bio-inspired materials, metalloids could push material science even further. Silicon and boron could pave the way for materials that self-heal or adapt to environmental changes.
-
Nanotechnology: Advances in nanomanufacturing are leveraging metalloids for ultra-precise applications, from medical devices to energy storage. Silicon nanowires are already being explored for next-gen batteries with higher energy density and faster charging times.
-
Lightweight composites: By combining metalloids with other materials, manufacturers can create parts that are lighter, stronger, and more heat-resistant than ever before. Boron carbide, a material harder than steel but much lighter, is already being used for cutting tools and armor, which could lead to more fuel-efficient vehicles and stronger, lighter aerospace components
The possible applications of these strange elements are vast, from creating better electronics to building more fuel-efficient vehicles. As we unlock their full potential, metalloids may push through the boundaries of innovation, driving us towards a world that is smarter, lighter, and more interconnected.
Get a quote
Ready to try metalloids in your manufacturing processes? Contact our team to explore custom manufacturing solutions that incorporate the materials you need to bring your project to life.